Summary
- Baricitinib/Olumiant COVID-19 results a plus.
- Pemazyre and other recent approvals should accelerate growth.
- Broader cancer pipeline makes Incyte a smart buy for longer-term investors.
Incyte (INCY) is an innovative pharmaceutical company that had its stock price peak at $140.11 in 2017 when it was considered to be a likely acquisition target. Most recently a drug it developed and licensed to Eli Lilly (LLY), Olumiant or baricitinib, showed promise as a treatment for COVID-19. While not to be neglected, this indication pales in significance to other therapies likely to increase Incyte's revenue and profits. Given its current commercially approved drugs, and its pipeline of potential therapies, I believe Incyte is currently priced attractively for longer term investors.
As of the close on September 17, at $90.35 per share, Incyte had a market capitalization approaching $20 billion. The 52-week low was $62.48, versus the 52-week high of $110.37. The most significant event affecting the stock in the last year was the approval of pemigatinib, now Pemazyre, in April 2020, for cholangiocarcinoma. I will cover that after assessing the COVID-19 news and Q2 2020 results. Then I will examine the rest of the pipeline.
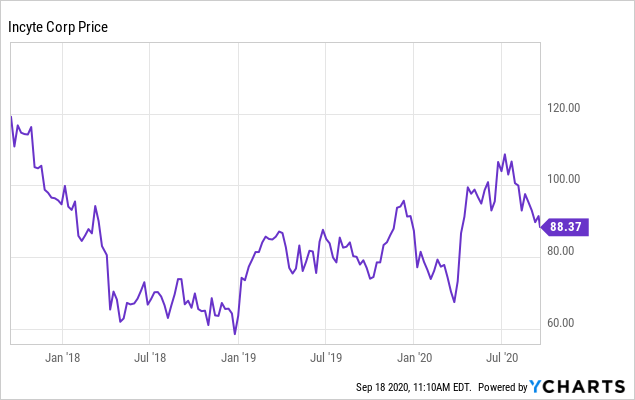 Data by YCharts
Data by YChartsOlumiant (Baricitinib) for COVID-19
Within pharmaceuticals, most investor attention has been focused on COVID-19 vaccines and therapies since the scope of the pandemic became clear. Many existing drugs are being tested to see if they might help. On September 14 Incyte announced positive results for Olumiant for COVID-19. Olumiant (baricitinib) is licensed by Incyte to Eli Lilly for the treatment of rheumatoid arthritis. It is an oral JAK inhibitor. In the trial sponsored by NIAID (National Institute of Allergy and Infectious Diseases) baricitinib plus remdesivir was compared to remdesivir alone in patients hospitalized with COVID-19. The combination met the primary endpoint of reduction of time to recovery. The reduction was about one day but was also statistically significant. It was a good study with over 1,000 patients, randomized and double-blinded. Full results will be published in a peer-reviewed journal.
What is not clear is if baricitinib works better than dexamethasone, a much less expensive drug. Lilly plans to discuss a possible emergency use authorization with the FDA. Even if it approved, I do not expect a significant revenue impact for Incyte. That could change if it takes longer than expected for a vaccine to become available, or if the number of hospitalizations rise dramatically. The bulk of the revenue will go to Lilly, only royalties will go to Incyte. On the other hand it may raise Olumiant's profile as a therapy for rheumatoid arthritis. Baricitinib is also in studies for atopic dermatitis, systemic lupus, and alopecia areata.






