Summary
- Little-known and thinly-traded busted IPO Verrica Pharmaceuticals Inc. filed an NDA for its candidate VP-102 in the treatment of molluscum contagiosum in September 2019.
- Approval, which appears likely, would be the first from the FDA for this $1 billion+ indication.
- The company also recently posted positive Phase 2 data for a second indication (common warts) and merited a deeper dive.
- Looking for a portfolio of ideas like this one? Members of The Busted IPO Forum get exclusive access to our model portfolio. Get started today »
Paris is a woman but London is an independent man puffing his pipe in a pub.”? Jack Kerouac, Lonesome Traveler
Today, we take an in-depth look at a thinly-traded dermatology concern as a response to an inquiry we recently had on this name from a SA follower.
Company Overview:
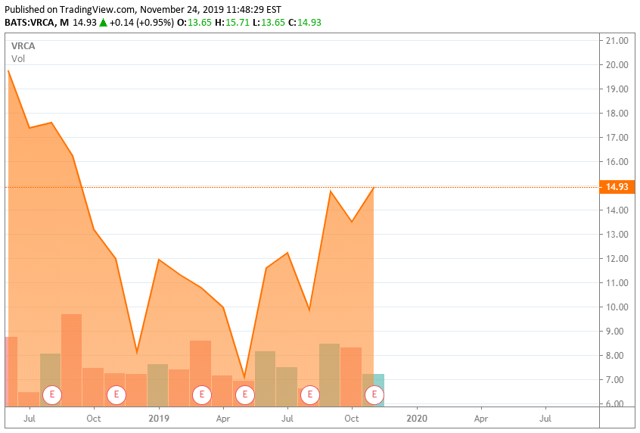
Verrica Pharmaceuticals Inc. (VRCA) is a West Chester, Pennsylvania, based clinical stage medical dermatology concern focused on the development of treatments for skin diseases. The company has one candidate being evaluated for three indications and will likely enter a second asset into the clinic during 4Q19. Verrica was founded in 2013 and went public in June 2018, raising net proceeds of $78.4 million at $15 per share. It currently trades slightly under its IPO price and commands a market cap just south of $380 million.
Pipeline: VP-102
The company’s lead asset is VP-102, a drug-device combination of topical solution cantharidin administered through a single-use precision applicator. Cantharidin is a chemical derived from the Blister Beetle and has been employed by dermatologists to treat warts and molluscum lesions for years. It is a painless liquid blistering agent that induces skin cells to release an enzyme that breaks the bonds that hold skin cells together. The resulting blister forces the wart or skin growth off the skin.
However, owing to its limited availability, inconsistent purity because it is not produced in accordance with good manufacturing practices, and lack of standardized administration, cantharidin has not been approved by the FDA. Current approved wart and molluscum lesion removal methods include cryotherapy, curettage, and laser surgery, all of which can lead to pain and/or scarring, making them somewhat unsuitable for children.
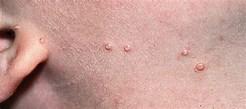 VP-102’s lead indication is for the treatment of molluscum contagiosum (molluscum), which is a common, highly contagious skin disease caused by the pox virus resulting in multiple raised flesh-colored papules (lesions). A typical outbreak presents 10 to 30 lesions, with severe cases presenting over 100. If left untreated, molluscum will normally resolve itself in about one year. It is passed by skin-to-skin contact and through contact with infected objects. It typically affects children ages 1 to 14 but can also affect adults with weakened immune systems. Verrica believes ~6 million American children are afflicted with molluscum with only ~1 million actually diagnosed annually, constituting a total addressable market north of $1 billion, with similar numbers in Europe.
VP-102’s lead indication is for the treatment of molluscum contagiosum (molluscum), which is a common, highly contagious skin disease caused by the pox virus resulting in multiple raised flesh-colored papules (lesions). A typical outbreak presents 10 to 30 lesions, with severe cases presenting over 100. If left untreated, molluscum will normally resolve itself in about one year. It is passed by skin-to-skin contact and through contact with infected objects. It typically affects children ages 1 to 14 but can also affect adults with weakened immune systems. Verrica believes ~6 million American children are afflicted with molluscum with only ~1 million actually diagnosed annually, constituting a total addressable market north of $1 billion, with similar numbers in Europe.